
2024年09月06日
Global marine microbial diversity and its potential in bioprospecting.The past two decades has witnessed a remarkable increase in the number of microbial genomes retrieved from marine systems. However, it has remained challenging to translate this marine genomic diversity into biotechnological and biomedical applications. Here we recovered 43,191 bacterial and archaeal genomes from publicly available marine metagenomes, encompassing a wide range of diversity with 138 distinct phyla, redefning the upper limit of marine bacterial genome size and revealing complex trade-ofs between the occurrence of CRISPR–Cas systems and antibiotic resistance genes. In silico bioprospecting of these marine genomes led to the discovery of a novel CRISPR–Cas9 system, ten antimicrobial peptides, and three enzymes that degrade polyethylene terephthalate. In vitro experiments confrmed their efectiveness and efcacy. This work provides evidence that global-scale sequencing initiatives advance our understanding of how microbial diversity has evolved in the oceans and is maintained, and demonstrates how such initiatives can be sustainably exploited to advance biotechnology and biomedicine. (Nature 2024, https://www.nature.com/articles/s41467-023-39706-9)
2023年11月22日
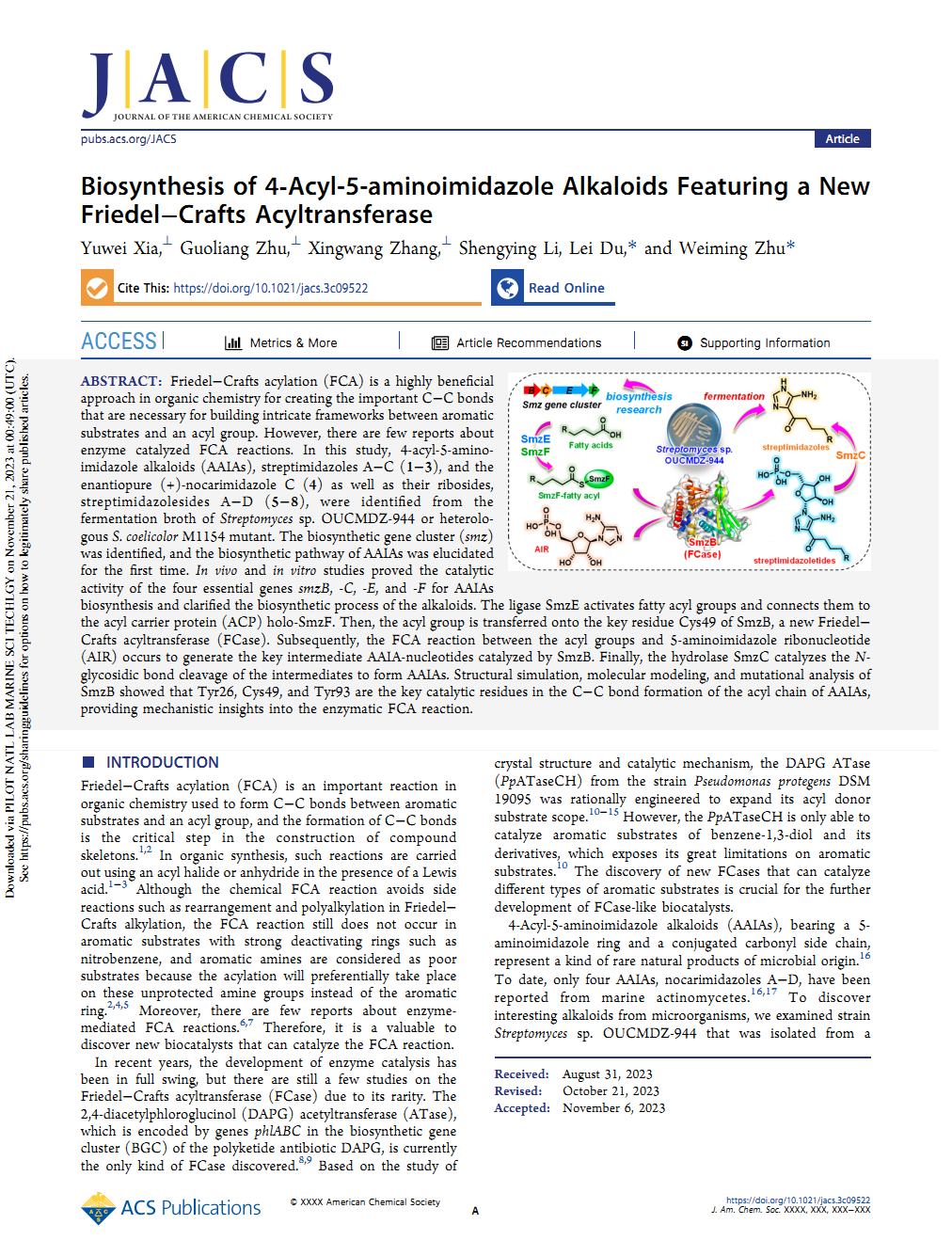
Biosynthesis of 4‑Acyl-5-aminoimidazole Alkaloids Featuring a New Friedel−Crafts Acyltransferase.Friedel−Crafts acylation (FCA) is a highly beneficial approach in organic chemistry for creating the important C−C bonds that are necessary for building intricate frameworks between aromatic substrates and an acyl group. However, there are few reports about enzyme catalyzed FCA reactions. In this study, 4-acyl-5-amino-imidazole alkaloids (AAIAs), streptimidazoles A−C (1−3), and the enantiopure (+)-nocarimidazole C (4) as well as their ribosides, streptimidazolesides A−D (5−8), were identified from the fermentation broth of Streptomyces sp. OUCMDZ-944 or heterolo-gous S. coelicolor M1154 mutant. The biosynthetic gene cluster (smz) was identified, and the biosynthetic pathway of AAIAs was elucidated for the first time. In vivo and in vitro studies proved the catalytic activity of the four essential genes smzB, -C, -E, and -F for AAIAs biosynthesis and clarified the biosynthetic process of the alkaloids. The ligase SmzE activates fatty acyl groups and connects them to the acyl carrier protein (ACP) holo-SmzF. Then, the acyl group is transferred onto the key residue Cys49 of SmzB, a new Friedel− Crafts acyltransferase (FCase). Subsequently, the FCA reaction between the acyl groups and 5-aminoimidazole ribonucleotide (AIR) occurs to generate the key intermediate AAIA-nucleotides catalyzed by SmzB. Finally, the hydrolase SmzC catalyzes the N-glycosidic bond cleavage of the intermediates to form AAIAs. Structural simulation, molecular modeling, and mutational analysis of SmzB showed that Tyr26, Cys49, and Tyr93 are the key catalytic residues in the C−C bond formation of the acyl chain of AAIAs, providing mechanistic insights into the enzymatic FCA reaction. (J. Am. Chem. Soc. 2023, https://pubs.acs.org/doi/10.1021/jacs.3c09522?fig=tgr1&ref=pdf)

2023年08月21日
Congratulations to Dr. Liu Kun on his return from the deep-sea dive aboard the “Jiaolong” submersible!On the afternoon of August 9, 2023, the “Deep Sea One” vessel, carrying the “Jiaolong” manned submersible and the entire scientific expedition team, successfully returned to the Qingdao National Deep Sea Base Pier, marking the successful completion of the first voyage of the 80th Chinese Ocean Expedition. Dr. Kun Liu, as a member of the scientific expedition team, participated in the entire voyage of the mission. On the day of docking, Prof. Shengying Li went to the Qingdao National Deep Sea Base Pier to welcome Liu Kun’s triumphant return.
This voyage set sail from Qingdao on June 16, 2023, and lasted for 55 days. Liu Kun made 238 dives, reaching a depth of 5076 meters, and successfully completed the meticulous observation and recording of the deep-sea seabed, retrieving valuable samples of deep-sea organisms and sediments. A total of more than 200 strains of bacteria, yeast, and filamentous fungi were isolated from deep-sea sources at the site.

2023年07月12日
Molecular insights into the catalytic promiscuity of a bacterial diterpene synthaseDiterpene synthase VenA is responsible for assembling venezuelaene A with a unique 5-5-6-7 tetracyclic skeleton from geranylgeranyl pyrophosphate. VenA also demonstrates substrate promiscuity by accepting geranyl pyrophosphate and farnesyl pyrophosphate as alternative substrates. Herein, we report the crystal structures of VenA in both apo form and holo form in complex with a trinuclear magnesium cluster and pyrophosphate group. Functional and structural investigations on the atypical 115DSFVSD120 motif of VenA, versus the canonical Asp-rich motif of DDXX(X)D/E, reveal that the absent second Asp of canonical motif is functionally replaced by Ser116 and Gln83, together with bioinformatics analysis identifying a hidden subclass of type I microbial terpene synthases. Further structural analysis, multiscale computational simulations, and structure-directed mutagenesis provide significant mechanistic insights into the substrate selectivity and catalytic promiscuity of VenA. Finally, VenA is semi-rationally engineered into a sesterterpene synthase to recognize the larger substrate geranylfarnesyl pyrophosphate. (Nat. Commun. 2023, https://www.nature.com/articles/s41467-023-39706-9)

2023年03月27日
Unnatural activities and mechanistic insights of cytochrome P450 PikC gained from site-specific mutagenesis by non-canonical amino acidsCytochrome P450 enzymes play important roles in the biosynthesis of macrolide antibiotics by mediating a vast variety of regio- and stereoselective oxidative modifications, thus improving their chemical diversity, biological activities, and pharmaceutical properties. Tremendous efforts have been made on engineering the reactivity and selectivity of these useful biocatalysts. However, the 20 proteinogenic amino acids cannot always satisfy the requirement of site-directed/random mutagenesis and rational protein design of P450 enzymes. To address this issue, herein, we practice the semi-rational non-canonical amino acid mutagenesis for the pikromycin biosynthetic P450 enzyme PikC, which recognizes its native macrolide substrates with a 12- or 14-membered ring macrolactone linked to a deoxyamino sugar through a unique sugar-anchoring mechanism. Based on a semi-rationally designed substrate binding strategy, non-canonical amino acid mutagenesis at the His238 position enables the unnatural activities of several PikC mutants towards the macrolactone precursors without any sugar appendix. With the aglycone hydroxylating activities, the pikromycin biosynthetic pathway is rewired by the representative mutant PikCH238pAcF carrying a p-acetylphenylalanine residue at the His238 position and a promiscuous glycosyltransferase. Moreover, structural analysis of substrate-free and three different enzyme-substrate complexes of PikCH238pAcF provides significant mechanistic insights into the substrate binding and catalytic selectivity of this paradigm biosynthetic P450 enzyme. (Nat. Commun. 2023, https://www.nature.com/articles/s41467-023-37288-0)
2021年09月18日
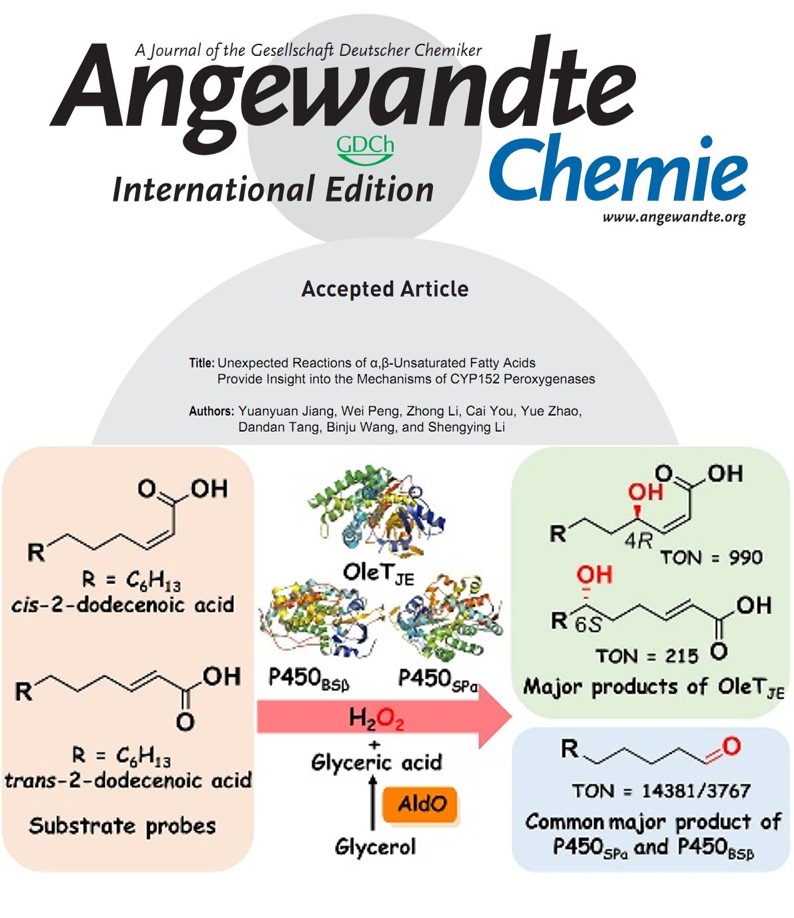
Our another new paper was published on Angew. Chem. Intl. Ed..Congratulations to the authors!Our new paper was published on Angewandte Chemie International Edition recently. In this study, we analyze the activities of three representative CYP152 peroxygenases (OleTJE, P450SPα and P450BSβ) towards cis- and trans-dodecenoic acids as substrate probes. The unexpected 6S-hydroxylation of the trans-isomer and 4R-hydroxylation of the cis-isomer by OleTJE, and molecular docking results suggested that the unprecedented selectivity is likely due to OleTJE 's preference of C2-C3 cis-configuration. More surprisingly, in addition to the common epoxide products, undecanal is the unexpected major product of P450SPα and P450BSβ regardless the cis/trans-configuration of substrates. The combined isotopically-labeled H218O2 tracing experiments, MD simulations and QM/MM calculations unravel an unusual mechanism for Compound I-mediated aldehyde formation, in which the active site water derived from H2O2 activation is involved in the generation of a four-membered ring lactone intermediate. These findings provide new insights into the unusual mechanisms of CYP152 peroxygenases. (Angew. Chem. Intl. Ed. 2021, https://onlinelibrary.wiley.com/doi/10.1002/anie.202111163.)
2021年09月18日
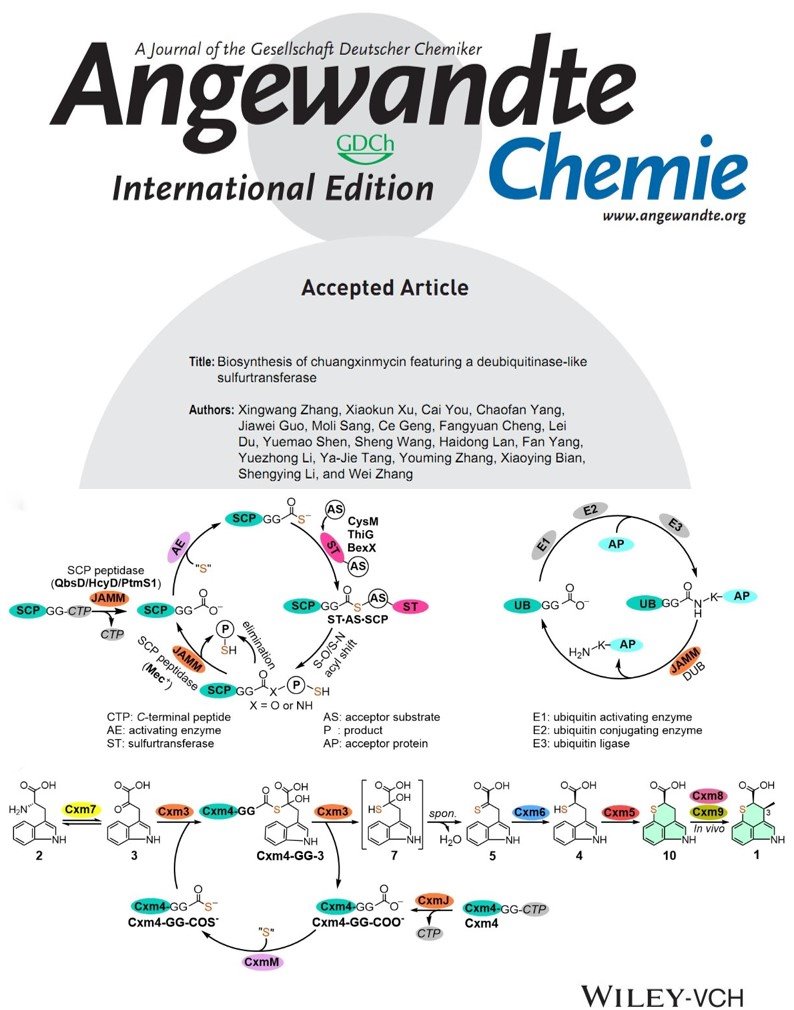
Biosynthesis of chuangxinmycin featuring a deubiquitinase-like sulfurtransferaseOur new paper was published on Angewandte Chemie International Edition recently. In this study, we resolved the central biosynthetic problem of enzymatic mechanism for sulfur incorporation by in vitro biochemical characterization of the key enzymes, and reconstitute the TPI skeleton in a one-pot enzymatic reaction. Particularly, we revealed that the JAMM/MPN+ protein Cxm3 functions as a deubiquitinase-like sulfurtransferase to catalyze a non-classical sulfur-transfer reaction by interacting with the ubiquitin-like sulfur carrier protein Cxm4GG. This finding adds a new mechanism for sulfurtransferase in nature. (Angew. Chem. Intl. Ed. 2021, https://onlinelibrary.wiley.com/doi/10.1002/ange.202107745.)
2021年09月06日
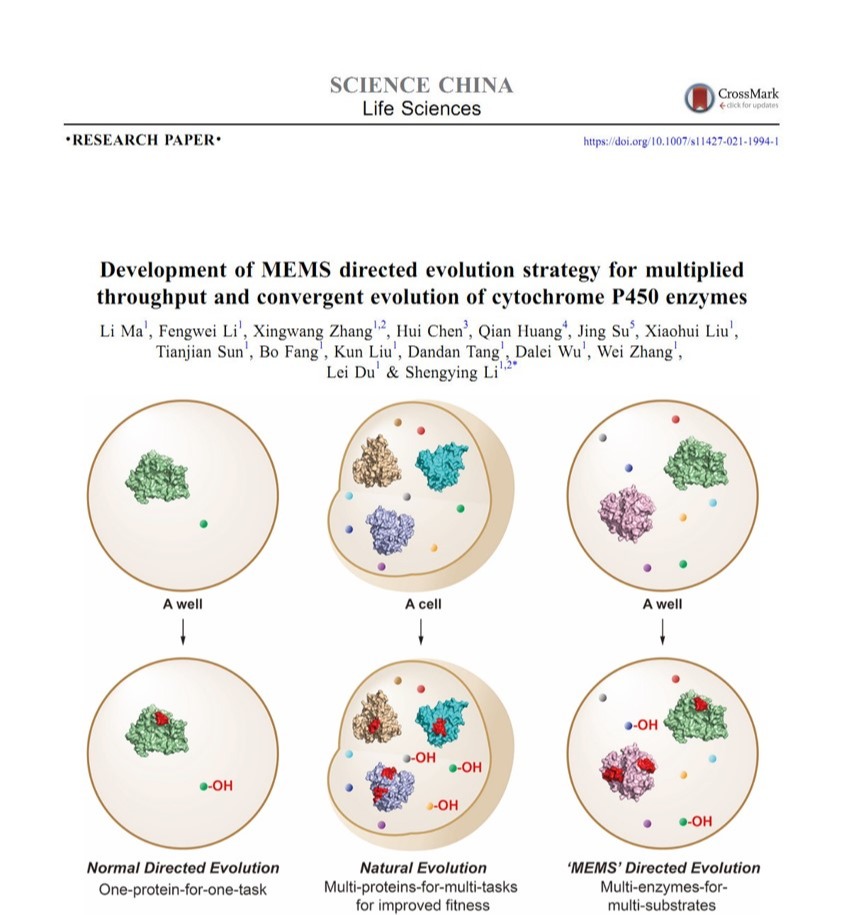
Our new paper was published on Science China Life Sciences. Congratulations to the authors!Recently, our new paper was published onScience China Life Sciences.In this study, we designed a novel “multi-enzymes-for-multi-substrates” (MEMS) DE model and establish the proof-of concept by running a NE-mimicking and higher-throughput screening on the basis of “two-P450s-against-seven-substrates” (2P×7S) in one pot. With the multiplied throughput and improved hit rate, we witnessed a series of convergent evolution events of the two archetypal cytochrome P450 enzymes (P450 BM3 and P450cam) in laboratory. It is anticipated that the new strategy of MEMS DE will find broader application for a larger repertoire of enzymes in the future. Furthermore, structural and substrate docking analysis of the two functionally convergent P450 variants provide important insights into how distinct P450 active-sites can reach a common catalytic goal. (Sci. China Life Sci.2021,https://doi.org/10.1007/s11427-021-1994-1.)

2021年05月26日
The public Open Day of the SKLMT held on May 22-25, 2021.The 2nd public Open Day of the State Key Laboratory of Microbial Technology (SKLMT) held on May 22-25, 2021 at Shandong University. Our group showed visitors the experiment of enzymatic degradation process of plastic film by the specific microbe, which enabled visitors to experience science at a short range.

2021年03月12日
Cytochrome P450 enzymes in fungal natural product biosynthesisOur new review was published on Natural Product Reports. In this review, we provide a comprehensive review to cover the most recent progresses from 2015 to 2020 on catalytic functions and mechanisms, research methodologies and remaining challenges in the fast-growing field of fungal natural product biosynthetic P450s. (Nat. Prod. Rep. 2021. https://pubs.rsc.org/en/content/articlelanding/2021/np/d1np00004g#!divAbstract)

2020年12月29日
Academic LectureProf. Jing Wu from State Key Laboratory of Food Science and Technology, Jiangnan University was invited to give a lecture of "New Technology of Industrial Enzyme Fermentation and Its Application" at Microbial Technology Institute of Shandong University.

2020年10月28日
2020 Annual Meeting of the Chinese Society for MicrobiologyOn October 23-27, 2020, the 2020 Annual Meeting of the Chinese Society for Microbiology was held in Chengdu. The theme of the meeting is microbiology and great health. A total of 13 teachers and students in our group attended the meeting. Prof. Wei Zhang was invited to give a lecture. In poster session, our group members displayed 9 posters and had positive discussion with the participants.

2020年06月07日
Welcome students back home !Starting from May 18, Shandong University students can begin returning to campus in batches. For the prevention and control of the epidemic situation on campus with students returning to school, SDU has formulated special work and emergency plans. At the same time, our lab will actively cooperate with SDU to do a good job in students management, including back-to-school approval, school entry, teaching and research arrangements, health monitoring, daily sanitation and disinfection, and laboratory management.

2020年05月19日
Fungal-derived brevianamide assembly by a stereoselective semipinacolaseRecently, our new paper was published on Nature Catalysisin collaboration with Prof. David Sherman’s group from University of Michigan and Prof. Robert Williams’s group from University of Oxford. In this study, we have identified the brevianamide A biosynthetic gene cluster from Penicillium brevicompactum NRRL 864 and elucidated the metabolic pathway. BvnE was revealed to be an essential isomerase/semipinacolase that specifies the selective production of the natural product. Structural elucidation, molecular modelling and mutational analysis of BvnE as well as quantum chemical calculations have provided mechanistic insights into the diastereoselective formation of the 3-spiro-ψ-indoxyl moiety in brevianamide A. This occurs through a BvnE-controlled semipinacol rearrangement and a subsequent spontaneous intramolecular [4+2] hetero-Diels–Alder cycloaddition. (Nat. Catal. 2020, https://doi.org/10.1038/s41929-020-0454-9)
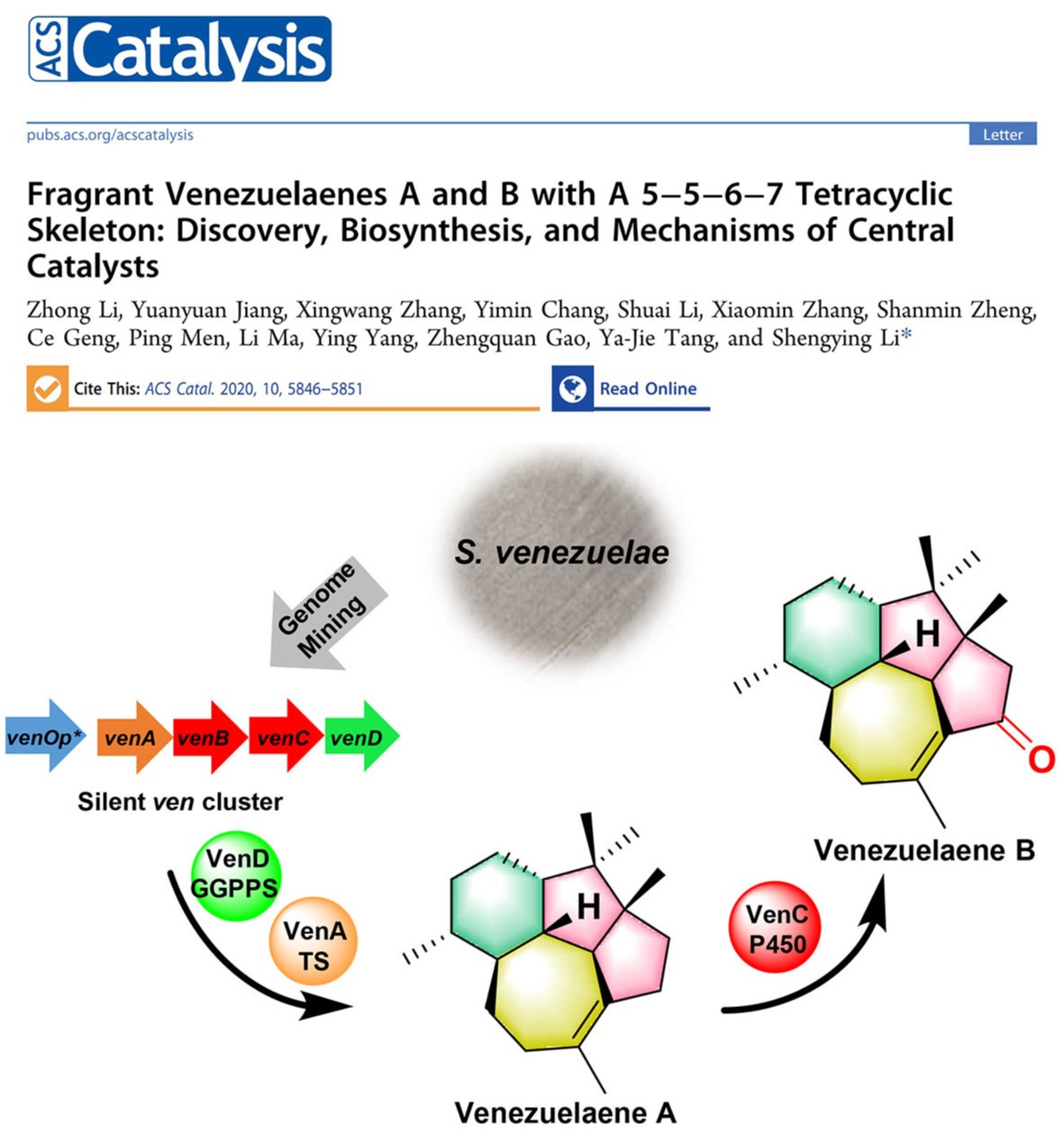
2020年05月06日
Fragrant venezuelaenes: Discovery, biosynthesis, and mechanisms of central catalystsOur new paper was published on ACS Catalysis. In this study, a cryptic terpenoid biosynthetic gene cluster ven was characterized from the model actinomycetic strain Streptomyces venezuelae ATCC 15439 through a genome mining approach. Silent gene activation and heterologous expression of ven led to the discovery of two diterpene metabolites venezuelaene A (1) and B (2, 5-oxo-venezuelaene A), both of which bear a 5−5−6−7 tetracyclic skeleton and show fragrance. A class I diterpene synthase VenA with an atypical 115 DSFVSD120 motif was revealed to catalyze the cyclization of geranylgeranyl pyrophosphate (GGPP) generated by the GGPP synthase VenD, giving rise to 1. The unique cyclization mechanism was elucidated by 13 C-tracer NMR experiments. The oxidative transformation of 1 into 2 was also characterized to be mediated by the cytochrome P450 enzyme VenC. (ACS Catal. 2020, 10, 5846-5851. https://pubs.acs.org/doi/10.1021/acscatal.0c01575)
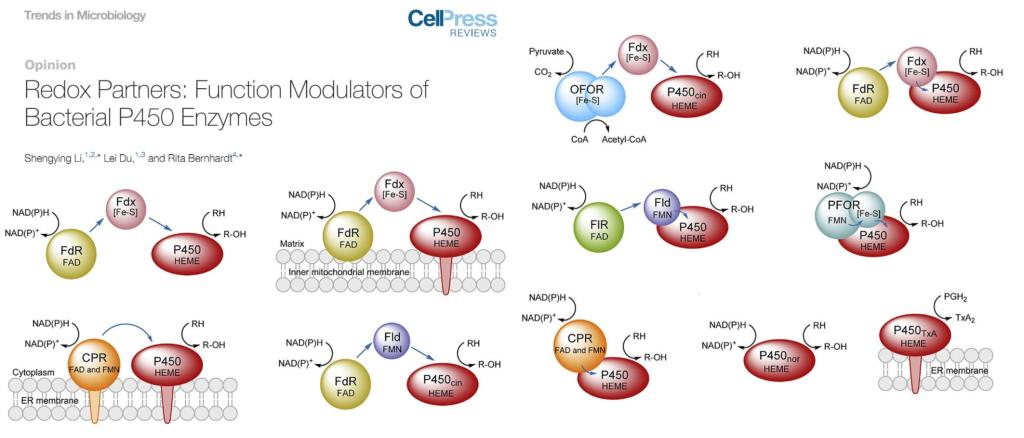
2020年03月18日
Opinion- Redox partners: Function modulators of bacterial P450 enzymesPer the invitation from Trends in Microbiology, we published an Opinion article entitled "Redox partners: Function modulators of bacterial P450 enzymes" together with Prof. Rita Bernhardt from Saarland University. This article introduced the arising trend to treat bacterial redox partners as important P450 function modulators in addition to the auxiliary electron transfer proteins. (Trends Microbiol. 2020. https://www.sciencedirect.com/science/article/pii/S0966842X20300482)
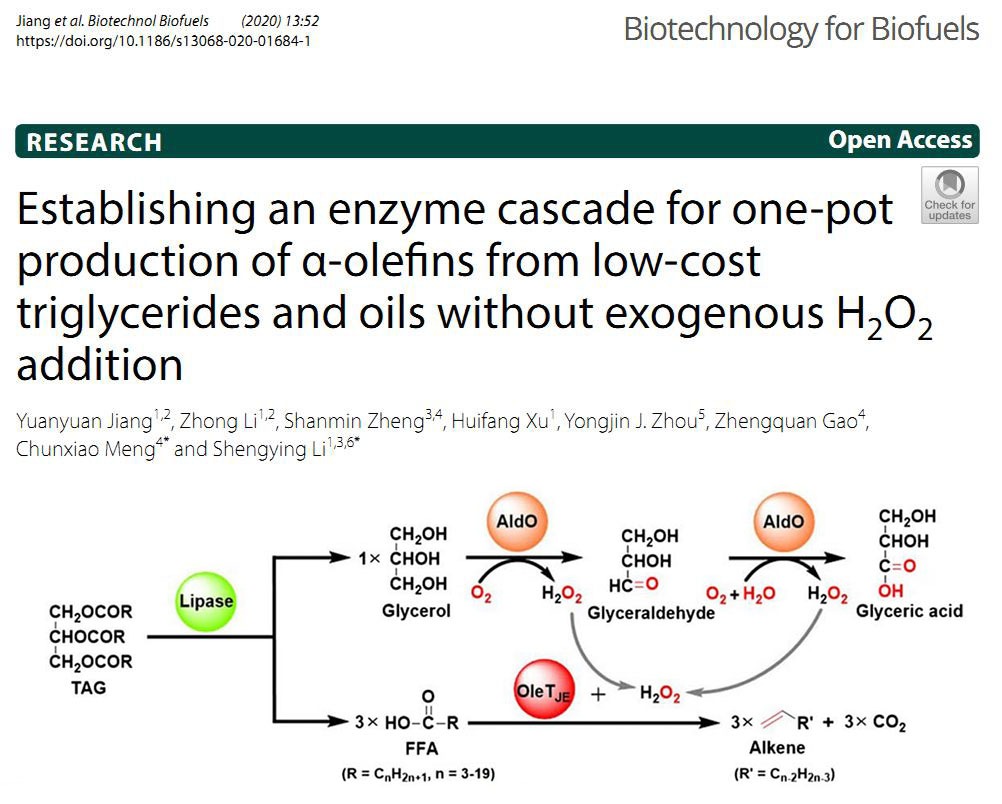
2020年03月16日
Establishing an enzyme cascade for one-pot production of α-olefnsOur new paper was published on Biotechnology for Biofuels. In this study, a novel enzyme cascade was developed for one-pot production of α-olefns from low-cost triacylglycerols (TAGs) and natural oils without exogenous H2O2 addition. This artificial biocatalytic route consists of a lipase (CRL, AOL or Lip2) for TAG hydrolysis to produce glycerol and free fatty acids (FFAs), an alditol oxidase (AldO) for H2O2 generation upon glycerol oxidation, and the P450 fatty acid decarboxylase OleTJE for FFA decarboxylation using H2O2 generated in situ. The multi-enzyme system was systematically optimized leading to the production of α-olefns with the conversion rates ranging from 37.2 to 68.5%. Furthermore, a reaction using lyophilized CRL/OleTJE/AldO enzymes at an optimized ratio (5 U/6 μM/30 μM) gave a promising α-olefn yield of 0.53 g/L from 1500 μM (~1 g/L) coconut oil. (Biotechnol. Biofuels. 2020, 12, 52. https://biotechnologyforbiofuels.biomedcentral.com/articles/10.1186/s13068-020-01684-1)

2020年01月08日
Academic LectureProf. Changning Liu from Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences was invited to give a lecture of "Long Non-coding RNA Studies - from Data Collection, Functional Prediction to Experimental Validation" at Microbial Technology Institute of Shandong University.
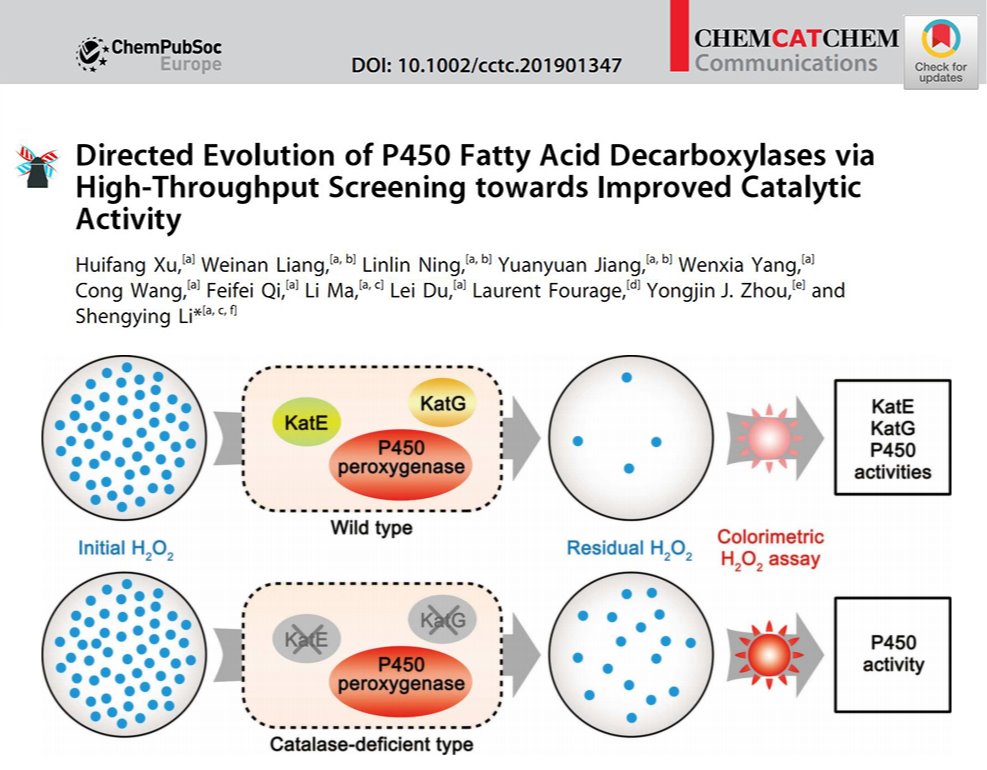
2020年01月08日
Directed evolution of P450 fatty acid decarboxylases via high-throughput screeningOur new methodology paper showed up on Chemcatchem. In this study, we devised a catalase-deficient Escherichia coli host strain and reported an HTS approach based on colorimetric detection of H2O2-consumption activity of P450 fatty acid decarboxylases (FADCs). Directed evolution enabled by this method has led to effective identification for the first time of improved FADC variants for medium-chain 1-alkene production from both DNA shuffling and random mutagenesis libraries. Advantageously, this screening method can be extended to other enzymes that stoichiometrically utilize H2O2 as co-substrate. (ChemCatChem. 2020, 12, 80-84. https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/cctc.201901347)

2019年12月27日
Academic LectureProf. Yazhong Xiao from Anhui University was invited to give a lecture of "Discovery of New Amylase" at Microbial Technology Institute of Shandong University.
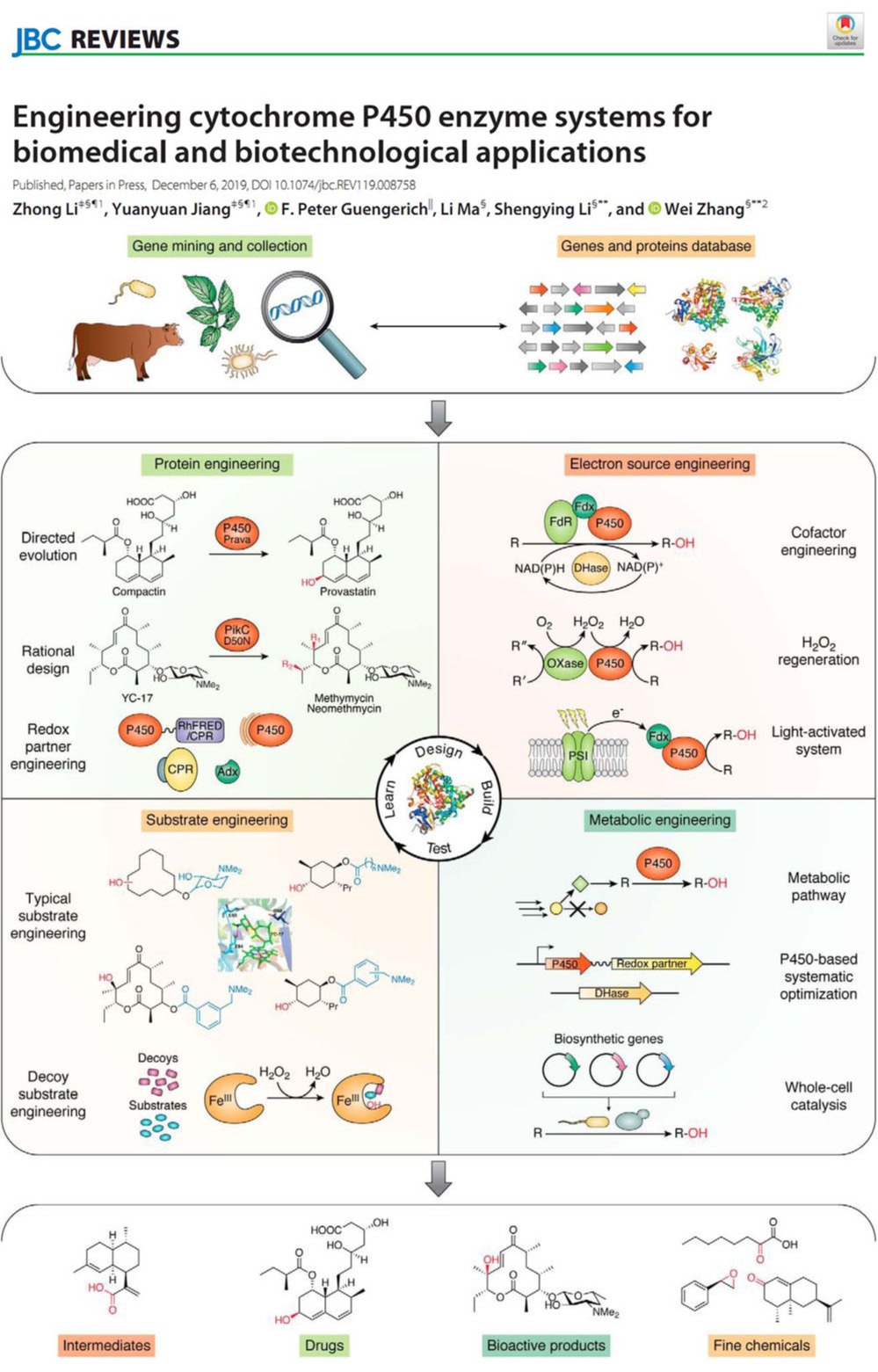
2019年12月06日
Engineering cytochrome P450 enzyme systems for biomedical and biotechnological applicationsA new review from this laboratory was published on the Journal of Biological Chemistry. In this review, we focused on recent progress of different strategies, including protein engineering, redox-partner engineering, substrate engineering, electron source engineering, and P450-mediated metabolic engineering, in efforts to more efficiently produce pharmaceuticals and other chemicals. We also discussed future opportunities for engineering and applications of the P450 systems. (J. Biol. Chem. 2020, 295(3), 833-849. https://www.jbc.org/content/295/3/833.full?sid=0c2d804f-d2a3-4f53-9b71-f6f6612ea810)

2019年11月15日
Congratulations to Prof. Shengying Li for receiving the ICBS-2019 Young Chemical Biologist AwardProf. Shengying Li was invited to attend The 8th Annual Conference of International Chemical Biology Society (ICBS-2019) at CSIR-IICT, Hyderabad, India during November 2-4, 2019. In this conference, Prof. Li was awarded the ICBS-2019 Young Chemical Biologist.
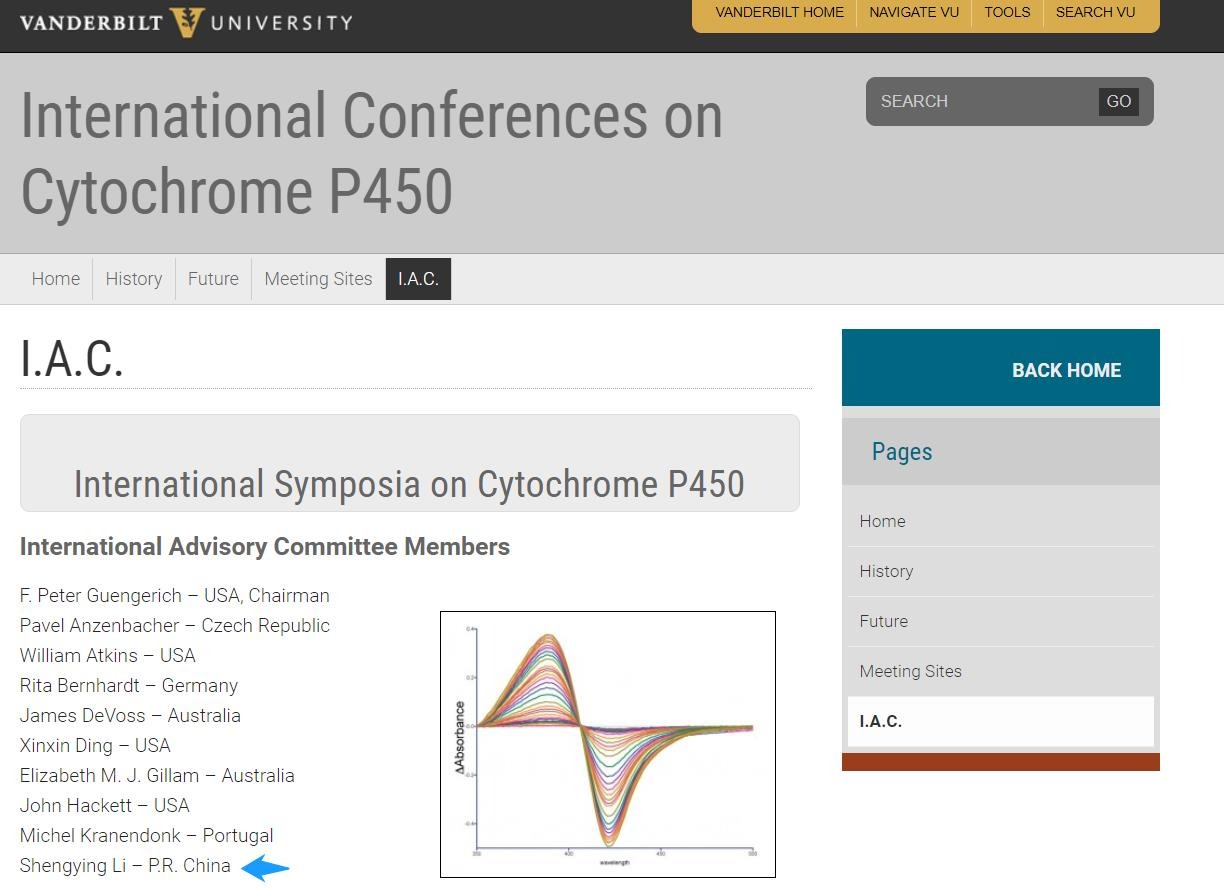
2019年10月31日
Prof. Shengying Li joined The International Advisory CommitteeProf. Shengying Li joined The International Advisory Committee for International Symposia on Cytochrome P450 (https://my.vanderbilt.edu/p450meetings/iac/)
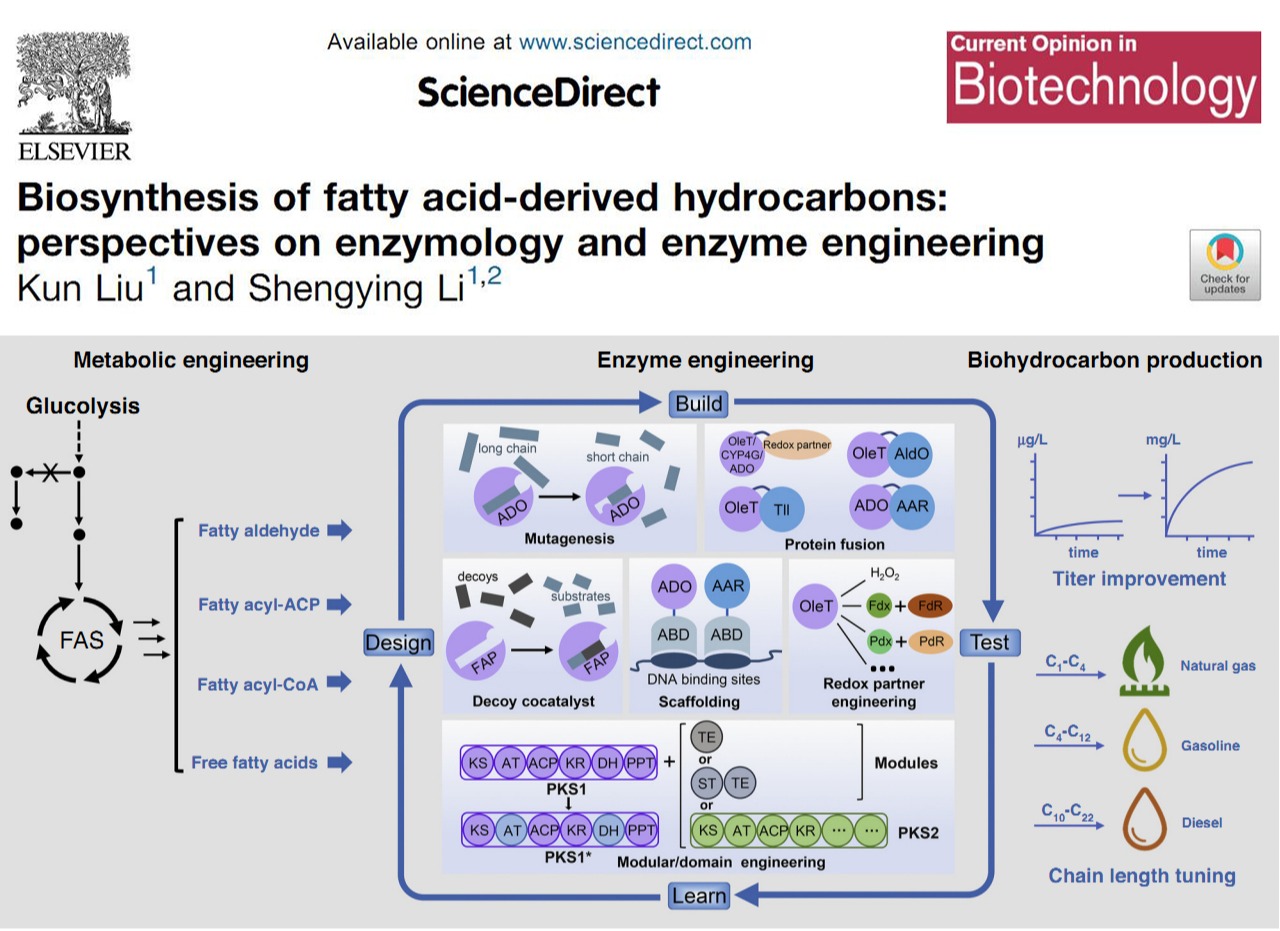
2019年09月17日
Biosynthesis of fatty acid-derived hydrocarbons: Perspectives on enzymology and enzyme engineeringEnormous fuel consumption and growing environmental concerns have been spurring development of renewable biofuels. Among various biofuels, fatty acid-derived biohydrocarbons are ideal alternatives to non-renewable fossil fuels due to their closest properties to petroleum-based fuels. In the past decade, novel hydrocarbon-producing enzymes have continuously been discovered and engineered. Recently, we published a review on Current Opinion in Biotechnology about the recent advances in biosynthesis of fatty acid-derived hydrocarbons with emphasis on enzymology and enzyme engineering, based on which some outlooks are provided. (Curr. Opin. Biotechnol. 2020, 62, 7-14. https://www.sciencedirect.com/science/article/pii/S0958166919300485)

2019年07月26日
The 4th Actinomycete Biology and the 2nd Microbial Drug Symposium was held in QingdaoFrom July 19-22, 2019, the 4th Actinomycete Biology and the 2nd Microbial Drug Symposium was held in Qingdao. The national meeting was co-sponsored by the Molecular Microbiology and Bioengineering Professional Committee of China’s Microbiology Society, Shandong Microbiology Society, and the State Key Laboratory of Microbial Technology, Shandong University. About 400 experts, scholars and students studying actinomycete biology, microbial drugs, and synthetic biology from all over the country attended the conference. The opening ceremony of the conference was presided by Prof. Shengying Li.
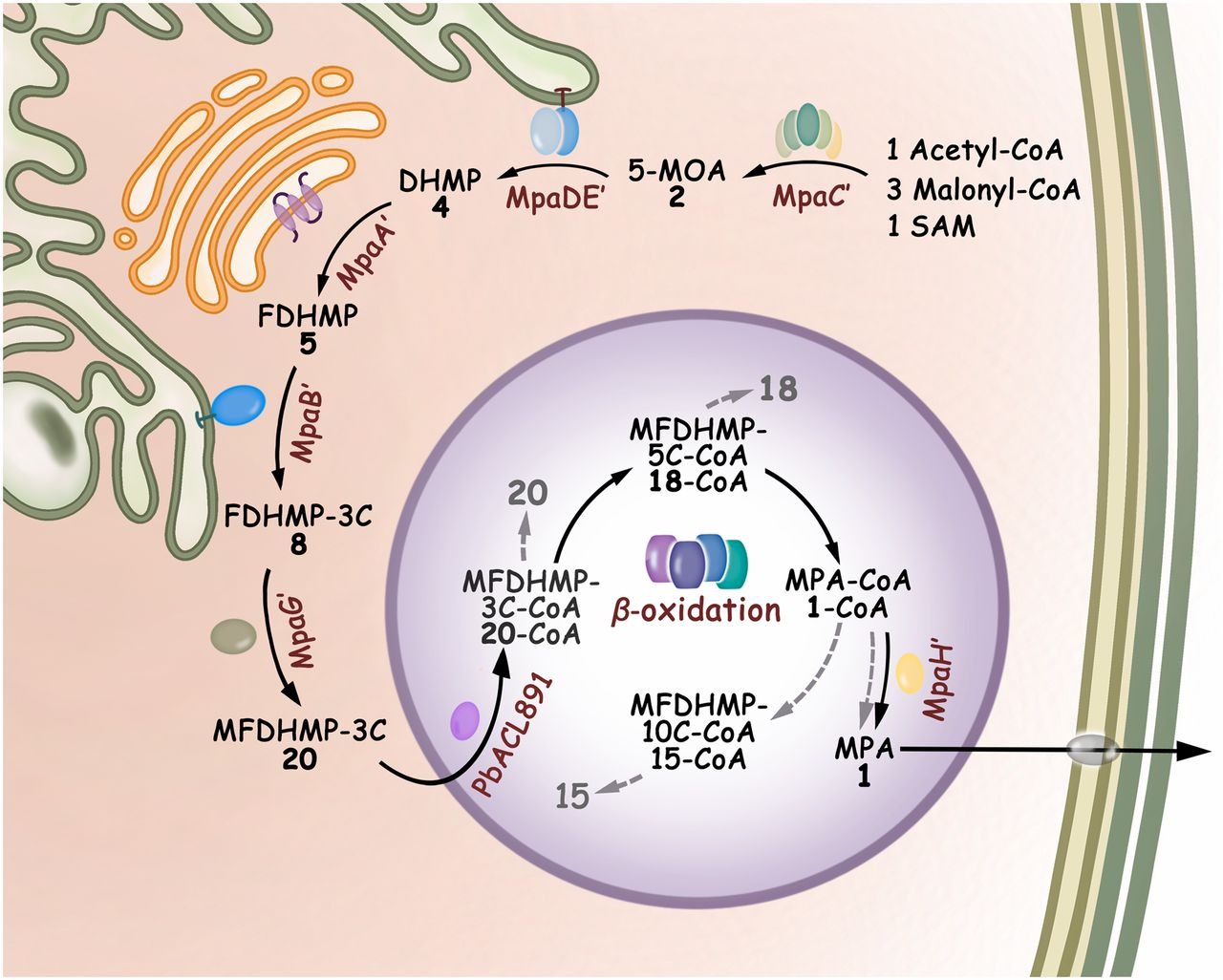
2019年06月05日
Compartmentalized biosynthesis of mycophenolic acidWe elucidate the full biosynthetic pathway of the fungal natural product mycophenolic acid (MPA). Besides the intriguing enzymatic mechanisms, we reveal that the MPA biosynthetic enzymes are elegantly compartmentalized; the oxygenase MpaB’ is the long-sought enzyme responsible for initiating the oxidative cleavage of the farnesyl side chain; and the subcellular localization of the acyl-coenzyme A hydrolase MpaH’ in peroxisomes is required for the unique cooperation between biosynthetic and β-oxidation catabolism machineries. This work highlights the importance of a cell biology perspective for understanding the underexplored organelle-associated essential catalytic mechanisms in natural product biosynthesis of fungi and other higher organisms. The insights gained in our study will benefit future efforts for both industrial strain improvement and novel drug development. (Proc. Natl. Acad. Sci. U.S.A. 2019, 116(27), 13305-13310. https://www.pnas.org/content/116/27/13305)

2019年04月08日
Biochemical characterization of three new α-olefn-producing P450 fatty acid decarboxylasesOur new paper was published on Biotechnology for Biofuels. In this study, we biochemically characterized three new P450 fatty acid decarboxylases including OleTJH, OleTSQ and OleTSA, with respect to their substrate specifcity, steady-state kinetics, and salt efects. These enzymes all act as an OleTJE-like fatty acid decarboxylase being able to decarboxylate a range of straight-chain saturated fatty acids (C8–C20) to various degrees. Site-directed mutagenesis analysis to the lower activity P450 enzyme OleTSA revealed a number of key amino acid residues within the substrate-binding pocket (T47F, I177L, V319A and L405I) that are important for delicate substrate positioning of diferent chain-length fatty acids and thus the decarboxylation versus hydroxylation chemoselectivity, in particular for the mid-chain fatty acids (C8–C12). In addition, the three new decarboxylases exhibited optimal catalytic activity and stability at a salt concentration of 0.5 M, and were thus classifed as moderate halophilic enzymes. (Biotechnol. Biofuels. 2019, 12, 79. https://biotechnologyforbiofuels.biomedcentral.com/articles/10.1186/s13068-019-1419-6)

2018年10月02日
Mechanistic insights into interactions between bacterial class I P450 enzymes and redox partnersWe constituted a P450 reaction matrix network with 16 Fdxs, 8 FdRs, and 6 P450s (against 7 substrates). By analyzing the reactivity profiles of 896 P450 reactions, together with phylogenetic analysis, redox potential measurements, structural simulations, and Fdx-P450 molecular docking, we provide important mechanistic insights into the recognition and interactions between bacterial Class I P450 enzymes and redox partners. (ACS Catal.2018, 8, 9992-10003. https://pubs.acs.org/doi/10.1021/acscatal.8b02913)